Development of Pulse Transient Chlorophyll Fluorometer
Bai Yu Hao Jianqing 1 2 2 Zheng Caixia Zhang Rong Rong Fu high 1 1
1. Teaching and Research Group of Plant Physiology, College of Biological Science and Technology, Beijing Forestry University 2. Beijing Yaxin Liyi Technology Co., Ltd.
The articles by Schreiber (2004) and Strasser (2004) represent the mainstream of current chlorophyll fluorescence instruments. Walz's PAM-modulated fluorometer was popularized by researchers in 1986 and became the mainstream of chlorophyll fluorescence kinetics. Subsequently, other companies have also introduced corresponding instruments. The non-modulated transient fluorometer overcomes the F 0 measurement problem due to the development of electronic component devices and single-chip data collectors. After 1990, Hansa's PEA and related new models mainly conform to the light suppression. Relevant research needs with non-reduction centers.
In recent years, transient fluorescence technology has been greatly developed, especially OJIP (or OKJIP) and other related research (Stasser 2001). Recently, due to the development of fluorescence kinetic models and related simulation techniques, simulation techniques based on transient fluorescence (Laisk et al., Photosynthesis in silico) have made new developments in transient fluorometers. However, the modulation fluorometer can calculate a variety of fluorescence kinetic parameters based on basic parameters such as F 0 , F m , F m ', F 0 ' and F s , which is still the main content of current chlorophyll fluorescence kinetics research. At the same time, we began to study the steady-state induced fluorescence kinetics from 2005, and it is possible to further develop the relevant parameters under post-steady state conditions. In view of the relatively simple non-modulated transient fluorometer, combined with the modulation of the saturation pulse technology, the Pulse Transition-Fluorescence Meter (trade name Yaxin-1161G chlorophyll fluorescence instrument) was developed with transient fluorometer characteristics. (It can measure OJIP and other parameters), combined with the advantages of modulated fluorescence (the ability to measure basic parameters such as F 0 , F m , F m ', F 0 ', and F s ), thus achieving portability and miniaturization.
First, the instrument related parameters
Saturation pulse intensity intensity range: 0-5000 μmol · photon · m -2 s -1
Photochemical intensity intensity range: 0-2000μmol · photon · m -2 s -1
Sample rate 5μs variable
A/D 12 bit
Second, the measurement results
1. Typical transient mode determination
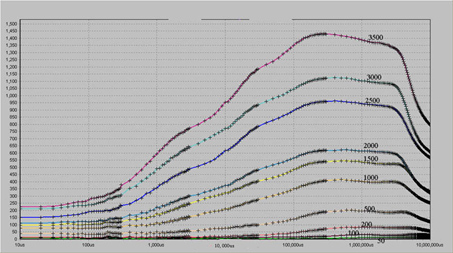
In this mode, the typical OJIP data can be obtained, and the OJIP curve and its related parameters under different actinic light intensity conditions are observed, as shown in Fig. 1.
Figure 1 OJIP curve under different actinic light intensity conditions
Note 1: Potted green radish was placed in the dark room, and the change of OJIP under different actinic light intensity was measured after dark adaptation for 2 hours. As shown in the above figure, the measured intensity is 3500, 3000, 2500, 2500, 2000, 1500, 1000, 500, 200, 100, and 50 μmol · photon · m -2 s -1 , etc. from top to bottom, and the duration of actinic light Both are 10s.
Table 1 Corresponding parameters measured under different actinic light intensity conditions
Measurement item | AL(T) | Total Time /s | F 0 | F m | F v /F m |
OJIP | 50(10) | 10 | 7 | 12 | 0.42 |
OJIP | 100(10) | 10 | 9 | 31 | 0.71 |
OJIP | 200 (10) | 10 | 16 | 81 | 0.8 |
OJIP | 500(10) | 10 | 39 | 203 | 0.81 |
OJIP | 1000(10) | 10 | 76 | 417 | 0.82 |
OJIP | 1500(10) | 10 | 113 | 582 | 0.81 |
OJIP | 2000(10) | 10 | 149 | 775 | 0.81 |
OJIP | 2500(10) | 10 | 167 | 971 | 0.83 |
OJIP | 3000(10) | 10 | 214 | 1124 | 0.81 |
OJIP | 3500(10) | 10 | 253 | 1423 | 0.82 |
2. Pulse transient fluorescence kinetics
In this mode, five parameters F 0 , F m , F m ', F 0 ' and F s can be obtained for the calculation of parameters related to the modulating fluorometer. At the same time, the OJIP pattern from the first saturation pulse after dark adaptation can be obtained, and the second pulse and the subsequent pulse-related changes, that is, the correlation changes of J and P, can be obtained. as shown in picture 2.
Fig. 2 OJIP curve under pulsed transient different actinic light intensity conditions
Note: Potted green radish was placed in the dark room, darkly adapted for 2 h, and subjected to measurement of pulse transient fluorescence kinetics under different actinic light intensities. As shown in the above figure, the measured intensities were 50, 100, 500, and 1800, respectively. During the measurement, the saturation pulse intensity is set to 5000, and the actinic light gradient is set. The measurement time is set by the instrument itself, the saturated light is 1 s, the actinic light is 9 s, for 10 s, and the measurement time is set to 300 s. At the end of the 300s measurement time, the long red light is applied for 4s, the dark time is 1s, and then a short saturation pulse appears to acquire F 0 ' , the whole measurement ends.
Table 2 Related parameters obtained by pulse transient fluorescence kinetics
measuring project | SL(T) | AL (T) | Total Time/S | FR T/S | Dark T/s | F 0 | F m | F v /F m | F 0 ' | F m ' | F s | qP | qN | ΦPSII |
Pulse transient | 5000(1) | 50(9) | 300 | 4 | 1 | 319 | 1536 | 0.79 | 303 | 1190 | 474 | 0.807 | 0.271 | 0.602 |
Pulse transient | 5000(1) | 100(9) | 300 | 4 | 1 | 273 | 1462 | 0.81 | 271 | 973 | 485 | 0.695 | 0.41 | 0.502 |
Pulse transient | 5000(1) | 200(9) | 300 | 4 | 1 | 361 | 2197 | 0.84 | 354 | 997 | 585 | 0.641 | 0.65 | 0.413 |
Pulse transient | 5000(1) | 300(9) | 300 | 4 | 1 | 383 | 1928 | 0.8 | 360 | 1015 | 604 | 0.627 | 0.576 | 0.405 |
Pulse transient | 5000(1) | 500(9) | 300 | 4 | 1 | 345 | 2161 | 0.84 | 347 | 914 | 593 | 0.566 | 0.688 | 0.351 |
Pulse transient | 5000(1) | 800(9) | 300 | 4 | 1 | 355 | 2100 | 0.83 | 306 | 677 | 530 | 0.396 | 0.787 | 0.217 |
Pulse transient | 5000(1) | 800(9) | 300 | 4 | 1 | 348 | 2205 | 0.84 | 294 | 601 | 525 | 0.248 | 0.835 | 0.126 |
Pulse transient | 5000(1) | 1000(9) | 300 | 4 | 1 | 342 | 2181 | 0.84 | 308 | 678 | 595 | 0.224 | 0.799 | 0.122 |
Pulse transient | 5000(1) | 1500(9) | 300 | 4 | 1 | 324 | 1819 | 0.82 | 285 | 542 | 492 | 0.195 | 0.828 | 0.092 |
Pulse transient | 5000(1) | 1800(9) | 300 | 4 | 1 | 316 | 1907 | 0.83 | 270 | 525 | 487 | 0.149 | 0.84 | 0.072 |
Pulse transient | 5000(1) | 2000(9) | 300 | 4 | 1 | 304 | 1880 | 0.84 | 268 | 509 | 478 | 0.129 | 0.847 | 0.061 |
3. Post-steady state induced fluorescence kinetics
After the steady state is reached, post-steady state induced fluorescence kinetics can be produced under the induction of actinic light due to the subsequent induction of saturation pulses, darkness, and far red light. As shown in Figure 3.
Figure 3 OJIP curve under steady state different actinic light intensity conditions




Note: Potted green radish was placed in the dark room, and dark-adapted for 2 h, and then measured under steady light intensity at different actinic light intensities. As shown in the above figure, the measured intensities were 50, 100, 500, and 1800, respectively. During the measurement, the saturation pulse intensity is set to 5000, and the actinic light gradient is set. The measurement time is set by the instrument itself, the saturated light is 1 s, and the actinic light is 10 s, for 11 s. Before the first saturated light, the blade is darkly adapted, and then the measurement is started, and the first saturated light is emitted. After the saturated light is completed, the far red light (4s), the darkness (1s), the actinic light (10s), and then another Saturated light, thus completing 2 measurements, that is, 2 saturated lights have appeared. In this experiment, 20 measurements were set, and the saturated light appeared 20 times during the whole measurement. The 20 saturated lights were separated by far red light, darkness and actinic light. After the last saturated light was completed, the measurement was completed.
Table 3 Related parameters obtained by post-steady-state fluorescence kinetics determination
Measurement item | SL(T) | AL(T) | Total T | FR Time/s | Dark T/s | F 0 | F m | F v /F m |
Post steady state | 5000(1) | 50(10) | 20 times | 4 | 1 | 365 | 1909 | 0.81 |
Post steady state | 5000(1) | 100(10) | 20 times | 4 | 1 | 356 | 2296 | 0.84 |
Post steady state | 5000(1) | 200 (10) | 20 times | 4 | 1 | 362 | 1859 | 0.81 |
Post steady state | 5000(1) | 500(10) | 20 times | 4 | 1 | 352 | 2024 | 0.83 |
Post steady state | 5000(1) | 800 (10) | 20 times | 4 | 1 | 382 | 1925 | 0.8 |
Post steady state | 5000(1) | 1000(10) | 20 times | 4 | 1 | 372 | 1918 | 0.81 |
Post steady state | 5000(1) | 1200(10) | 20 times | 4 | 1 | 357 | 1908 | 0.81 |
Post steady state | 5000(1) | 1200(10) | 20 times | 4 | 1 | 338 | 1750 | 0.81 |
Post steady state | 5000(1) | 1800(10) | 20 times | 4 | 1 | 325 | 1649 | 0.8 |
Post steady state | 5000(1) | 2000(10) | 20 times | 4 | 1 | 246 | 1412 | 0.83 |
4. Determination of the correlation between cyclic electron transport and linear electron transport
After steady-state fluorescence, if the actinic light is stopped, P 680 stops the electron output, and P 700 continues to transmit electrons in the far red light. It is the photosynthetic electrons entering the cyclic electron transfer. After about 10s or longer, there is a sudden photochemistry. Induction causes P 680 to emit electrons. In this process you can understand the situation of electronics. As shown in Figure 4.
Figure 4 OJIP curve for different far red light durations
references
Schreiber U.Chlorophyll Fluorescence: a Signature Photosynthesis.pp279-319. Eds by Papageorgiou GC, Govindjee. Springer (2004).
Strasser, RJ Ibid. Pp 321-362.
Test date: July 2011
Test site: Beijing Forestry College of Science and Technology
Instrument manufacturer: Beijing Yaxin Liyi Technology Co., Ltd.
This project Gannan quality navel orange whole fruit (not peeled) as raw materials, using self-developed patent Orange surface sterilization technology to maximize retained a whole pieces of all Orange essence (Orange: Shun Qi, phlegm, pulp: fiber laxative, fruit seeds: allergic inflammation, juice: whitening detox).
Conventional enzyme production is mainly produced by hand, hand-sliced, hand-fermentation, the company every step of the process are used self-developed technology and equipment to automate production. Moreover, the conventional enzyme production have adopted a fermentation technology, enzyme often taste discomfort, sour and other issues, I produced using a variety of enzymes, probiotics composite grade fermentation technology, fermentation at different times with different strains, and two or three times through fermentation, enzymes such pure taste, streptozotocin balanced nutrients. Scientific and standardized process:
I produced using enzymes navel cleaning, sterilization, slice, fermentation (fermentation grade composite multi-strain), solid-liquid separation, bottling and other steps. Each step developed a patented automated production equipment, fully automated production, to avoid the traditional manual production workers are not standardized, easy to pollution, low production efficiency, product quality is unstable defects.
Companies registered capital of 35 million yuan, the end of 2014 the total assets of 48.69 million yuan, including fixed assets of 37.52 million yuan. The company's existing cooperation Orange cultivation base 7043.5 acres, the company production base is located in Jiangxi County Tech Industrial Park Chu Tan industrial area, covers an area of 120 acres, it has built a standard plant 9,000 square meters, Nissan 6000 kg Orange enzymes and other liquid enzyme products. Enzyme, known as enzyme, refers to a polymer substance having biocatalytic functionality. In the catalytic reaction system an enzyme, the reactant molecules are known as substrates, enzyme substrates by catalytic conversion to another molecule. Almost all cellular activity of enzymes involved in the process are required to improve efficiency. Similar to other non-biological catalysts, enzymes chemical reactions by lowering the activation energy to accelerate the rate of the reaction, most of the enzyme catalyzed reaction rate can be increased a million times; in fact, the enzyme is to provide an activation energy needs than another low way, so that more particles to have less than the activation energy of the reaction kinetic energy, thus speeding up the reaction rate. Enzyme as a catalyst, in itself is not consumed during the reaction, it does not affect the chemical equilibrium reactions. Positive enzyme catalysis, but also a negative catalytic effect, not only to accelerate the reaction rate, but also to reduce the reaction rate. And other non-living catalysts is different, having a high degree of specificity of enzyme, only a catalytic reaction or produce a particular specific configuration.
Orange Enzyme Solution
Orange Enzyme Solution,Enzyme Essence Liquid,Fresh Orange Fermentation
Ganzhou Green days Biochemical Technology Co., Ltd. , https://www.tlqcjs.com