1> Dental saliva ejector Description
1. Length: 150mmÂ
2. Non rusting alloy wire (brass coated)Â
3. Clear, colorsÂ
4. CE0197, ISO13485:2003, ISO9003:2008,FDA,FSC
Â
Facilitates uninterrupted fluid and light debris removal and other extended use. Rust-proof alloy copper materials can be bent into the shape of a variety of needs; Soft and smooth mouth design, the prevent suck organization, safe and comfortable; Non-toxic tasteless green soft materials and It is disposable to prevent cross infection
Â
Features:
1. Smooth and round tip help to enhance patient comfort while protecting delicate mucosal tissue
2. Non-rusting alloy wire (brass coated),optimized fluid removal
3. Facilitates uninterrupted fluid and light debris removal
4. Allows for extended use
5. Color: clear with blue tip, clear with clear tip, white with white tip, colors with color tip.
Â
Â
| Material | Non rusting alloy wire (brass coated) |
| Size | 150mm, |
| Color | clear with blue tip, clear with clear tip, white with white tip, color with color tip |
| Packaging | 100pcs/bag,10bag/ctn carton size: 31.5X24X16 cm G.W.: 4.8KG 20' container: 2300 cartons. |
| Delivery | 7-15 days after receipt of deposit. |
| Terms of payment | L/C,T/T, Western Union, Paypal |
| Note | 1.This  product is sterilized by ethylene oxide 2.Validity:2  years 3.Disposable |
| Certification | CE, ISO9001, ISO13485 |
| OEM | 1. Material or other specifications can be according to customers' requirements. 2. Customized Logo/brand printed. 3. Customized packaging available. |
1.Good safety of gelatin-free.The first lyophilized Varicella Vaccine, containing no gelatin from animals, invented and produced in China. Getting rid of gelatin from varicella vaccine can significantly decrease the ratio of anaphylaxis incidence .
2.Long validity period by good stability. The first approved varicella vaccine with 36 months of validity period in the world. Adopting BH-2 stabilizer with own IP rights (Chinese patent granted No.: ZL200910138411.6, International patent application No.: PCT/CN2009/001405) greatly enhances the stability of the product and ensures the validity period for 36 months under the storage condition.
3.Better protection with high titer and immune eff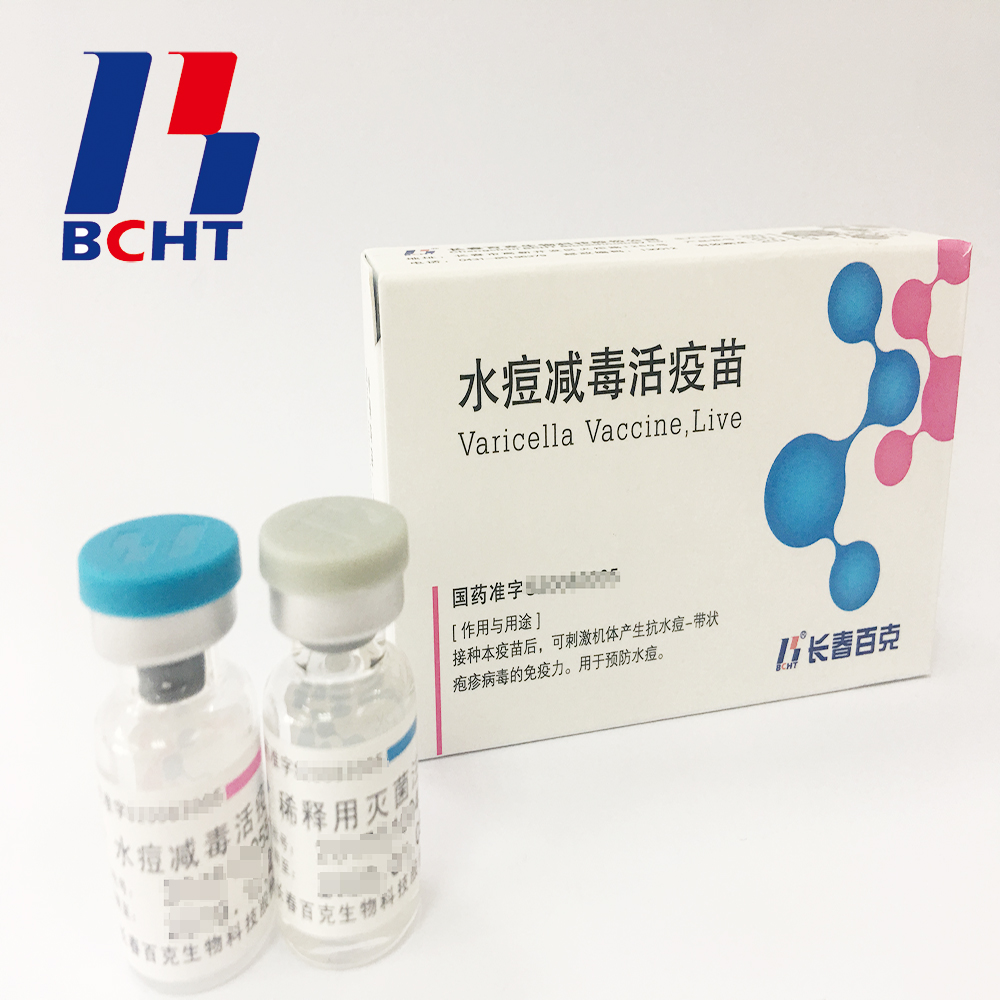 icacy. It`s documented that compared with the varicella vaccine of low titer, high titer vaccine can reduce breakthrough cases by nearly 75% in vaccination. Of all released batches of Varicella Vaccine from BCHT, the titers are not less than 10000 PFU/dose tested by National Institutes for Food and Drug Control.
icacy. It`s documented that compared with the varicella vaccine of low titer, high titer vaccine can reduce breakthrough cases by nearly 75% in vaccination. Of all released batches of Varicella Vaccine from BCHT, the titers are not less than 10000 PFU/dose tested by National Institutes for Food and Drug Control.
Varicella Vaccine(Live)
Live Bioproducts Pharmaceutical,Live High Potency Medicine,Live Preventive Pharmaceutical,Varicella Biopharmaceutical Live
Changchun BCHT Biotechnology Co. , http://www.ccbcht.com