Oral Escherichia coli for liver disease, microbial therapy completed the first dose
April 03, 2018 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Today, Synlogic, based in Cambridge, Mass., announced that the company's SYNB1020 was administered to the first patient in a recent phase 1b/2a clinical trial of hyperammonemia.

Hyperammonemia is a metabolic disorder characterized by excess ammonia in the blood. Generally, ammonia is produced as a by-product of protein metabolism and a microbial degradation product of a nitrogen-containing compound in the small intestine. The ammonia is then converted to urea in the liver and excreted with the urine. However, if the liver is damaged, whether it is due to congenital genetic defects or liver damage caused by acquired liver disease, it will cause excessive ammonia accumulation in the blood, which may cause brain poisoning, hospitalization or irreversible brain damage, and even death. There are approximately 33,000 cases of hereditary hyperammonemia in the United States, and up to 900,000 cases of hepatic encephalopathy due to impaired liver function.
SYNB1020 is an orally available E. coli-based synthetic organism. The company uses synthetic biology tools to program natural microbes so that it can compensate for dysfunctional metabolic pathways in the gut and regulate microbes to regulate metabolism. There are about 100 large bacterial populations in the human gut, which have different biochemical active libraries, such as synthetic vitamins B and K, metabolic bile acids, sterols and xenobiotics. Although the function of the gut flora is constantly being discovered, many scientists have begun to view the gut flora as an additional human organ.
On the other hand, the use of synthetic biology for editing intestinal flora to transfer part of human organ function or to create new metabolic pathways is likely to produce a safe and well tolerated therapeutic bacterium. If this idea is proven in Synlogic's recent clinical trials, it will open up a whole new field of drug design and pharmacokinetics.
The 1b/2a clinical trial conducted this time is divided into two parts. The first part was initially tentatively performed in a group of patients with cirrhosis and an open-label group with an end-stage liver disease model (MELD) score of less than 12. They will receive 6 days of SYNB1020 during which they will be hospitalized for a running-in diet, assessment, safety monitoring and collection of blood, urine and stool samples. Once the safety and tolerability of the drug has been determined, proceed to Part II. The second part was a randomized, double-blind, placebo-controlled trial of patients with cirrhosis and hyperammonemia. The steps will follow the first part. The primary end point of the study is still to test safety and tolerability, but will also study the effect of the drug on plasma ammonia levels. It is expected that there will be a line of data by the end of this year.
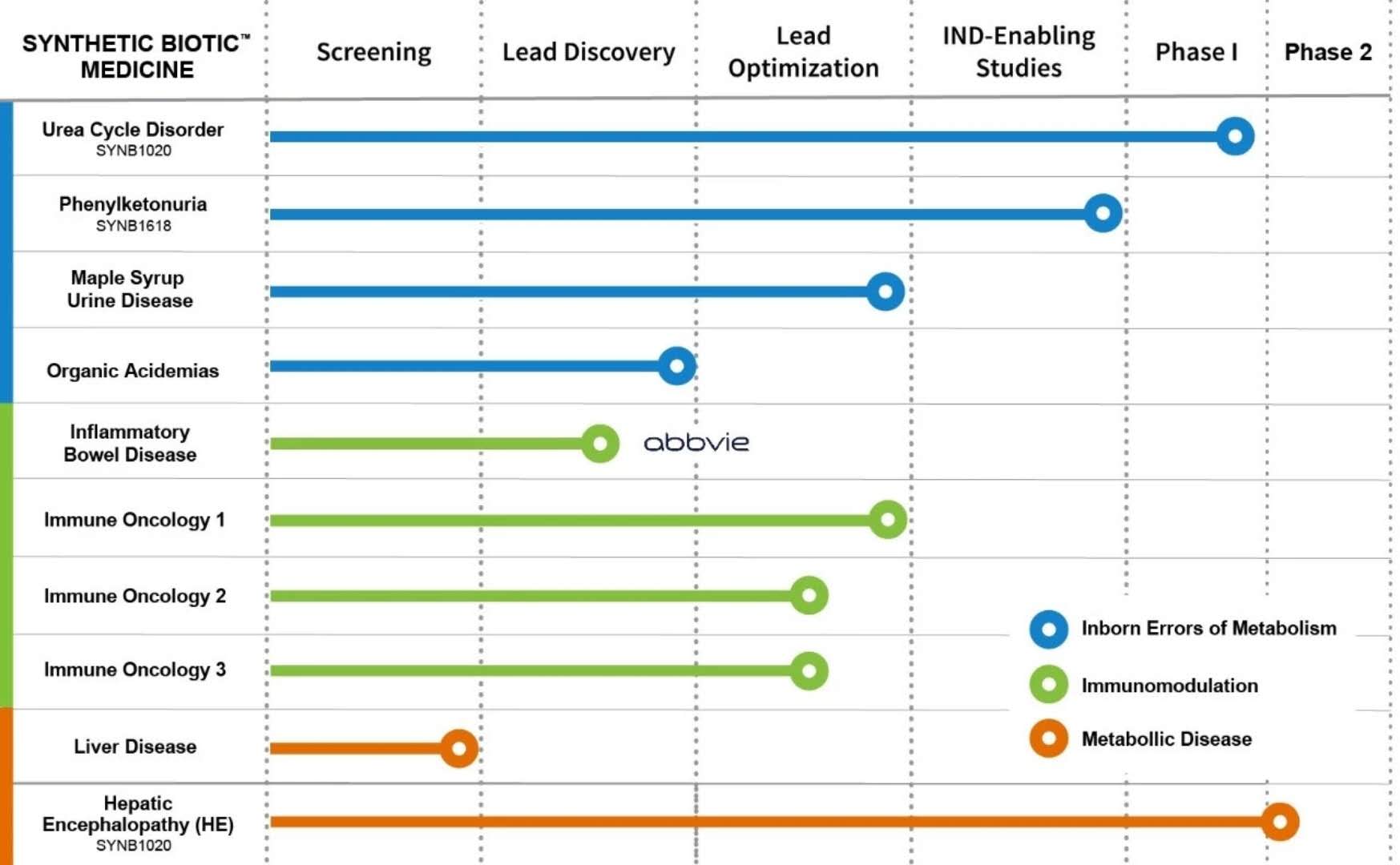
â–² Synlogic R & D pipeline (Source: Synlogic official website)
“We recently reported that the first phase of the SYNB1020 clinical trial showed that this synthetic biopharmaceutical is well tolerated and provides evidence of dose-dependent mechanisms consistent with the design of healthy volunteers,†Aoife Brennan, Chief Medical Officer, Synlogic Ms. said in a statement: "We look forward to assessing the safety, tolerability and therapeutic potential of SYNB2010 in patients with liver cirrhosis. We are excited about the potential of SYNB2010 in this indication, which will also Provide more medical options for patients with chronic liver disease."
"In 2018, we have the conditions to continue to advance our platform. As clinical trials of synthetic biopharmaceuticals SYNB1020 and SYNB1618 begin to treat hyperammonemia and phenylketonuria (PKU), we are likely to be at the end of the year, in two Verification of our conception in patients with different diseases," Dr. JC Gutierrez-Ramos, President and CEO of Synlogic, said in a statement. "These data will be key to determining whether SNBB1020 and SYNB1618 can help patients control their disease and demonstrate the potential of our synthetic biology platform."
Reference materials:
[1] Engineered Microbiome Company Synlogic Doses First Patient in Liver Disease Trial
[2] Synlogic official website
A Foot Spa Machine with heat is a device used to provide a relaxing and therapeutic foot massage. It usually has a basin filled with warm water and has various massage settings such as vibration, bubbles, and rollers. The heat function helps soothe tired and aching feet, while the massage setting provides a deep-tissue massage that helps improve circulation, reduce tension, and relieve pain. Some foot spas also come with removable attachments, such as pumice stones and brushes, for extra exfoliation and cleansing. Overall, a foot spa machine with heat is a great tool for anyone looking to pamper their feet and promote overall relaxation and wellness.
Foot Spa Machine With Heat,Bubble Foot Bath Massager,Foot Massage Machine,Pedicure Foot Spa Machine
Huaian Mimir Electric Appliance Co., LTD , https://www.footspamachine.com