Small manufacturing equipment and laboratory equipment confirmation
Example analysis GAMP 5
Manufacturing Equipment and Laboratory Equipment Balances: Software Category
summary:
Practitioners hold the operating manual in the hands of the GAMP 5 template, which will use computer-supported systems in a GMP environment for risk reasons. Depending on the software thus categorized, performing a computer system validation is more or less costly.
Practitioners hold the operating manual in the hands of the GAMP 5 template, which will use computer-supported systems in a GMP environment for risk reasons. Depending on the software thus categorized, performing a computer system validation is more or less costly.
Today there are few manufacturing and laboratory equipment that are not known for digital and software support technologies. Therefore, this essay will focus on the software used in small devices. The name of the software is called "embedded software".
summary:
GAMP 5 is a hands-on guide that enables engineers and quality managers to provide risk-based, computer-system validation strategies for GMP production. Because of the risk-based validation software, it takes more time and higher costs.
Today, most manufacturing and laboratory equipment is based on systems for computer operations. This short article will focus on some of the software in small devices; often referred to as "embedded software."
Key words:
GAMP 5, small manufacturing equipment, small laboratory equipment, risk, software category, template, balance
1. Introduction
Weighing locations (analytical and laboratory balances, container balances, weighing balances, screening control balances) were found in many process steps in the production and quality control of the pharmaceutical industry. Because they exist in almost every production and quality control, they are ideal for considering the practicality of GAMP 5.
They are generally classified in small manufacturing equipment and laboratory equipment, although they play a full role in production and quality in the process chain. Hint: For the following GAMP 5 considerations, we exclude 100% of our software-programmable control systems or software-implemented weighing technology solutions in PC solutions, nor can they be classified as small devices.
Separate, unconnected balances on the data side further lose their importance, and today it is standard to connect to the system via (bidirectional) data transmission. This may involve simple data backup, but it also includes two-way data fusion in complex control processes. Default values ​​for process data traceability will result in data associations and automatic data backups (out of paper). Of course, you must consider the access rights during operation (operational phase) and setup/calibration (installation and calibration phases). However, today's access to hierarchical levels has been satisfactorily resolved even in the balance.
The dependent GAMP 5 category will be found depending on the use and assembly of the balance (software).
Category classification should be performed for reasons in the above balance instance. Based on the existing GAMP 5 guidelines, appropriate computer system validation will be endorsed (Appendix 11 of the GMP Operation Manual).
2. The balance in progress
Two very typical use cases for pharmaceutical production and quality control of the balance will be described below .
2.1 Example Analysis 1 :
Figure 1 : Balance as a stand-alone device, software category 3 ( Sartorius Visualization)

- The balance will be considered an " independent " device. This means that the device is not connected to any system that transmits data for reprocessing.
- The example is: a balance in quality control.
- The balance is equipped with business software that runs on standard hardware components. According to GAMP5 , these balances will display " Unconfigured products - with GAMP software category 3 , hardware category 1" .
- These balances can be used in research and development, production or analysis / quality control.
è Balance will be regarded as “ Original Equipment (OTS) †.
No computer confirmation is required. Data migration is not possible. There are design specifications (URS) and requirements specifications. Fully implement IQ/OQ and support through risk analysis.
2.2 Example Analysis 2 :
Fig. 2 : Balance, connected to the system, software category 3 ( Sartorius visualization)

- The balance is considered here together with the temporary data storage and transmission in the system.
- They are connected to the system. The prerequisite is that the superior system will be subject to computer confirmation.
- Unique data processing and long-term data storage do not occur in the balance.
- Integration of business software that can run on standard hardware components into the balance. This means that these balances will show " Unconfigured products - with GAMP software category 3 , hardware category 1" according to GAMP5 .
- These balances can typically be used in research and development, production or analysis / quality control.
Example: a chemical additive balance in a clean room; a balance with a barcode reading connection
è The balance can be viewed as a black box system. No computer confirmation is required. There are design specifications (URS) and requirements specifications. Fully implement IQ/OQ and support through risk analysis. Additional data interface detection (ensuring process data migration, the best, documented assurance can be achieved through sample surveys).
3. General operation method
After selecting the device, the attributes of the process connection are considered for confirmation (this is not the subject, for example selecting a balance according to the URS default value, according to the minimum required net weight):
1. Check the supplier's QM system
2. Risk analysis
3. Create user technical documentation
3.1 Check the supplier's QM system
GAMP 5 Category 3 emphasizes 'products' (see GAMP 5, Section 7.1.1). In the summary, the Special Representative emphasized Category 5 of the 'Customized Application'. In all cases it will be advisable to use the installed manufacturer's QM system to check the transparency of the process (see GAMP 5, section 7.3). This 'product' must be installed in the QM process of the supplier (manufacturer) (in all cases).
A feature of the development software is that the vendor's proof of the techniques and methods of software development is based on a suitable practice (see GAMP 5, Table 7.1). The software must be controlled and organized according to the quality and safety system, as it is not possible to perform quality "entry testing" of the software.
For suppliers of balances ('products') used in GMP-related environments, the following risk considerations apply not long ago:
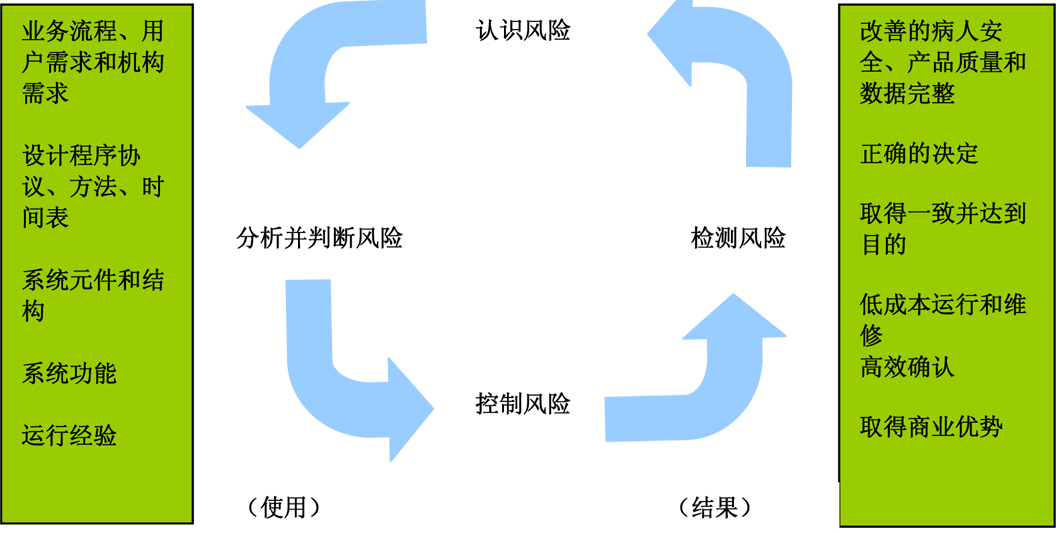
Figure 3 : Using a suitable GMP computer-supported system based on risk (from the GAMP 5 Operating Manual (ISPE) )
It is also important to note that the product can only be put into the market if it clearly meets the needs of the target group and can be justified (here: used in the pharmaceutical industry).
3.2 Quality Risk Management
General practice experience consisting of the types of analysis listed in GAMP 5 will be displayed from the supplier's perspective:
- Basic analysis based on available information (including reference materials)
- Postal audit using questionnaires
- On-site audit
The ratio of postal audit to on-site audit is approximately 10:1. The reason is that the user's risk considerations apply to small manufacturing equipment and laboratory equipment, and this applies to the balance, which will result in less risk. Vendors will be supported through the best use of modular software. A certificate must be submitted.
In order to avoid the effects, the balance in progress does not play a GMP-related role: it plays the role of in the net weight (as a platform balance or container balance), in quality control (as an analytical balance or laboratory balance), as Metering balance (topping, precise dose), in the production of tablets (weight of tablets) or as a screening balance with control functions (packaging area). Not long ago users assigned it as a GMP-related one.
3.3 quality comes from design
In general, European legislation focuses on the integration of quality standards and their integration in the design of equipment (Lit. ICH Q 10, Appendix 20 of the EU GMP Operation Manual).
As mentioned in Figure 1, one of the characteristics of the development software is that the techniques and methodologies used are based on the appropriate practice (see GAMP 5, Table 7.1).
GMP risk analysis Technical documentation information:
|
Figure 4 : Balance Risk Analysis Technical Documentation (Sartorius AG)
As a support, the appropriate supplier will provide users with product-related risk analysis or templates (eg risk analysis purification, risk analysis software). They are minimally used to reduce the temporary cost of the project team and, if appropriate, 100% applied to the user's technical documentation. A successful risk analysis should be used as a suitable pre-filter for the following costs. Traceability of qualification tests for risk analysis is ensured through a traceability matrix.
Here, the individual case considerations of the user and the results of the document policy play a decisive role.
3. 4 users – files
In general, the content and scope of risk-based, relevant identification materials will be recommended in GAMP 5 (Lit. EU-GMP-Operational Manual 15, 20; ICH Q 8, 9, 10; GAMP 5).
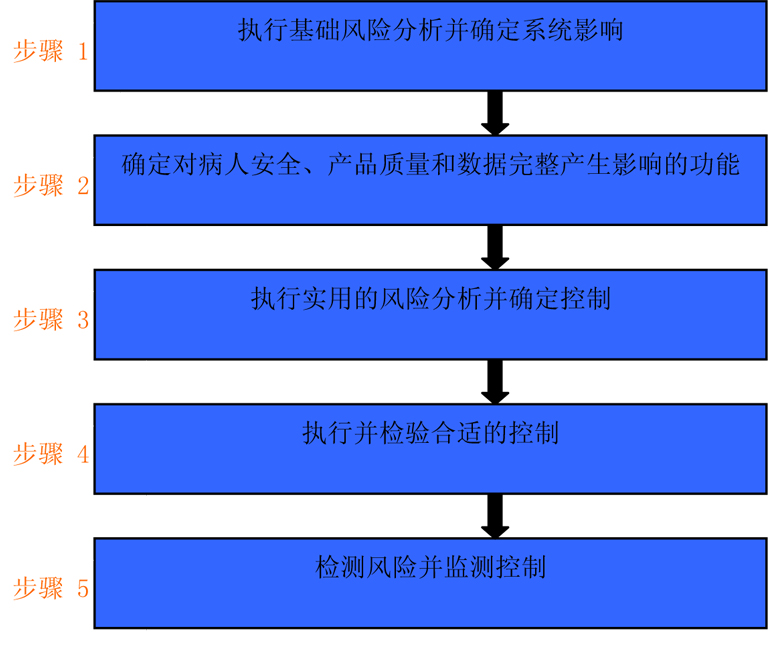
Figure 5 : Using a suitable GMP computer-supported system based on risk (from the GAMP 5 Operating Manual (ISPE) )
For small manufacturing equipment in software category 3, it is meaningful to summarize steps 1 through 3 (inset 5).
Action possibilities based on risk and applicable to different system categories are shown in Section 7 of the GAMP 5 Operation Manual:
About Category 3 (unconfigured products):
"Can cover up to Category 3 of traditional products in a single analysis of all relevant risks, so as shown in the illustration M3.6 can be determined for a particular system, the need for additional analysis and make appropriate plans." (Source From: GAMP 5)
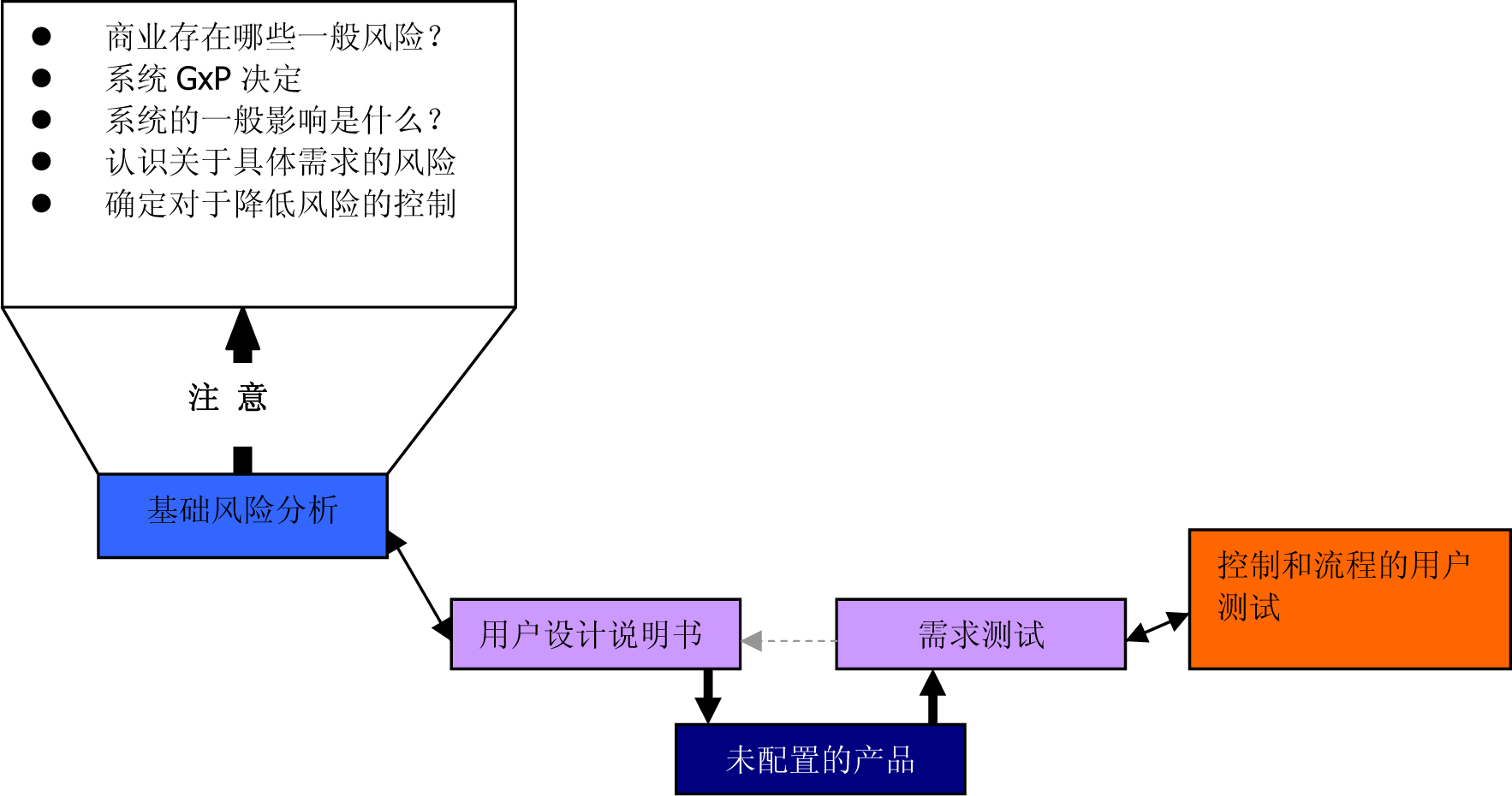
Figure 6 : Using a suitable GMP computer-supported system based on risk (from the GAMP 5 Operating Manual (ISPE) )
Basic risk template for balances in software category 3
Selected analysis matrix:
W = probability of failure:
Frequency, how many failures may occur, relative to the total amount.
W | Percentage estimate | point |
impossible | Less than 1% | 1 |
medium | Up to 50% | 2 |
high | Above 50% | 3 |
A = impact on product quality or patient/transporter
A | point |
Almost no effect | 1 |
Moderately serious failure | 2 |
Extremely serious failure | 3 |
E = probability of discovery, affected:
• The number of controls (during production, delivery, inspection, ...)
• Controlled costs
E | Percentage estimate | point |
high | Above 50% | 1 |
medium | Up to 50% | 2 |
impossible | Less than 1% | 3 |
RPZ = risk priority number (analysis)
Calculate the risk priority number (RPZ) according to the formula:
Risk Priority Number ( RPZ ) = ( W ) x ( A ) x ( E ) |
After calculation, the range of priority numbers for each risk is:
1   ≤ RPZ ≤ 27 .
The risk priority number can be entered as a result in the GMP threshold. In any case, it should be marked whether it involves GMP criticality risk (effective from RPZ ≥3). Which risk priority numbers are classified as “critical,†“acceptable,†or “non-critical†will depend on the selected level of the risk priority range. Therefore, users must understand the impact on patient safety before defining a risk priority range that is classified as “critical,†“acceptable,†or “non-critical.â€
Classified as a critical risk priority, remedies must be defined and translated.
These measures may be occupied tests and processes that control risks. It also includes organizational measures such as regulations, training, etc. or system changes. The goal has always been to control risk. After passing the measures, the risk will be analyzed again to show the risks that are now controllable.
Excerpt from a risk analysis template for a balance:
Numbering | Function / object | Potential failure | analysis | GMP criticality | Measure / annotation | analysis | GMP criticality | ||||
W | A | E | W | A | E | ||||||
15 | Computer confirmation | No or incorrectly determined, do you need a computer confirmation? | H L | Determine if you need a computer confirmation | |||||||
Fault inquiry | H | ||||||||||
Although not required, computer confirmation was not performed | |||||||||||
Failure result | L | ||||||||||
Non- GMP compliant jobs | |||||||||||
Numbering | Function / object | Potential failure | analysis | GMP criticality | Measure / annotation | analysis | GMP criticality | ||||
W | A | E | W | A | E | ||||||
16 | access permission | Unauthorized users cannot use assigned features | H L | Determine access to standard software and possibly use these features | |||||||
Fault inquiry | H | ||||||||||
Unable to determine access rights | |||||||||||
Failure result | L | ||||||||||
Unreasonable data exchange | |||||||||||
Figure 7 : Excerpt from the Balance Risk Analysis Technical Document ( Sartorius AG – Founder : Steinbeis Transferzentrum Drugs – Medical Equipment – Cosmetics )
The risk technical documentation is covered in detail in this article. This is consistent with the risk-based use highlighted in GAMP 5. Of course, the manufacturer will provide all required standard technical documentation (manufacturer certificate and product certificate, technical specification/data page, transporter manual (SOP) and DKD/USP measurement log). This service is available from every qualified manufacturer and is accompanied by service life related services (calibration, maintenance).
In the case study 1 : 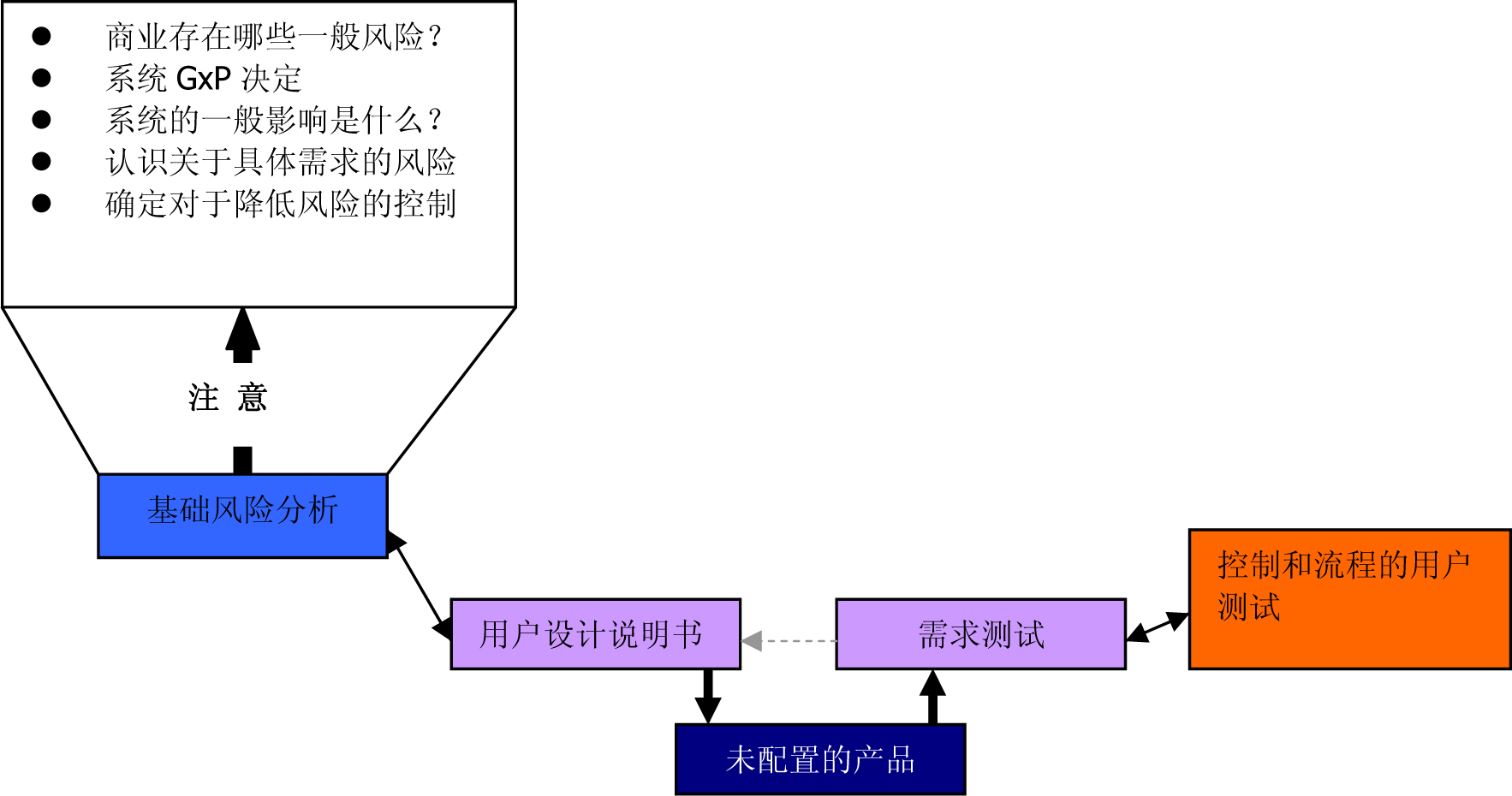
Figure 8 : Using a suitable GMP computer-supported system based on risk (from the GAMP 5 Operating Manual (ISPE) )
No computer confirmation is required here because it involves "standalone" devices. Data migration is not possible.
Fully implement IQ and OQ and support through risk analysis. There should be a design specification followed by a requirement specification. Shows how to convert the requirements from the design specification.
In the case study 2 :
See Figure 8.
As an extension of the sample analysis 1, the data interface is checked to ensure data migration. This allows for the best and documented assurance through sample surveys.
Source code detection is not performed.
If IQ and OQ are fully implemented in the sample analysis 1, and supported by risk analysis, there should also be a design specification and a requirement specification in the sample analysis 1.
4. Last note:
The supplier's QM system is also important in small equipment, such as balances. With the prepared supplier audit and supplier technical documentation application, the cost can be significantly reduced in the early stages. In addition, manufacturers must take care of and take up the “quality by design†add-on, as in general, the “entry test†of quality takes risks.
As a balance for small manufacturing equipment and laboratory equipment, classification is not complicated compared to other systems and equipment. The cost can be confirmed based on the GAMP 5 recommendations (based on their respective risk considerations!) and the 'cost less' mode allowed by GAMP 5. In addition, risk templates and technical documentation provided by manufacturers (as experts) are supported, with reduced time and expense.
Technical documents:
Good automated production practices, version 5 (GAMP5), ISPE 2008.
European Commission, Business and Industry: EU guidelines on good practices in the production of human and veterinary medicine, Part 1 – Basic requirements for pharmaceutical products, Appendix 15 “Eligibility and Validationâ€, July 2001.
European Commission, Business and Industry: EU guidelines on good practices in the production of human and veterinary medicine, Part 1 – Basic requirements for pharmaceutical products, Appendix 20 “Quality Risk Managementâ€, March 2008.
ICH Q 8, Drug Development: November 2008.
ICH Q 9, Quality Risk Management: November 2005.
ICH Q 10, Drug Quality System: June 2008.
Writer :
Professor Ingrid M ü ller      ( HS Albstadt-Sigmaringen , University of Technology Technical University Course )
Elke Weber Master of Engineering         ( HS Albstadt-Sigmaringen , University of Technology Technical University Course )
Hartmut Meier Master of Engineering       ( IHM Hartmut Meier Engineering Bureau , Engen )
Klaus Thornagel Master of Engineering     ( Director ISPE DACH )
Ingolf Popel Vice President of Marketing/Sales/Services ( Sedolis Scientific Instruments (Beijing) Co., Ltd. )
For more information and in-house training on small laboratory equipment risk analysis in the GAMP5 environment, please contact us (e-mail: document.write('') ssil. document.write('') )
Spice Novelties Co.,Limited , https://www.wholesale-adult-toys.cn