After numerous revisions, the "Opinions of the Central Committee of the Communist Party of China and the State Council on Deepening the Reform of the Medical and Health System" finally passed away in the past, and the medical reforms that carried the national welfare were once again on the journey. On April 22, as the only professional forum focusing on medical electronic technology and solutions in China, China International Medical Electronic Technology Conference (CMET 2009) was held on the occasion of the implementation of the new medical reform. Many experts, scholars and leading enterprises in the medical electronics industry gathered in Shenzhen. Sharing the development trend of the medical electronics market and technology in the new medical reform, as well as the latest technology and design concepts in the medical electronics industry, the participating companies expressed their hope to participate in China's medical reform with their own strength, and share this with the community. A historical mission.
New medical reform, new journey, medical electronics industry has a long way to go
The new medical reform pays more attention to the application of electronic information technology. The program specifically proposes to establish a practical and shared medical and health information system nationwide. It can be foreseen that in this wave of medical reform, the informationization of the medical system will be Will lead the new round of rapid growth in the medical electronics market.
Li Shuzhen, general manager of CCID Consulting's Semiconductor Research Center, pointed out that under the new medical reform, the demand for medical electronic equipment in rural and second- and third-tier cities and communities will be released quickly, and the different characteristics of the home and medical market will also make China's portable medical electronics. The market is “fast and stableâ€. It is expected that China's portable medical electronics market will continue to grow at a rate of around 30% in the next three years. Portable medical electronics manufacturers face enormous opportunities. 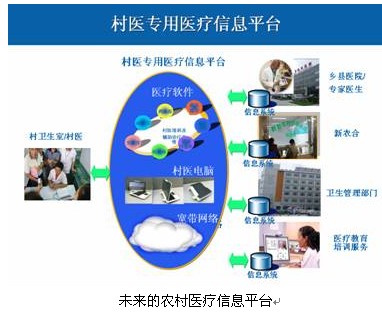
However, Dr. Wang Xiaoqing, a member of the Expert Committee of the China Medical Device Industry Association, who was later debuted, reminded everyone that although the medical electronics market looks beautiful, the road ahead will be extremely bumpy, and the gap between China's medical electronics industry and foreign countries is gradually expanding in recent years. . In order to gain a share of this market opportunity, manufacturers need to pay more attention to the research on the characteristics of medical electronics, research and development systems and models, market supervision and access threshold, and can grasp the whole process and management of medical electronic products. Dr. Wang Xiaoqing advised everyone that although the medical electronics market is still technology-led, among the factors that determine its market development, technical power only accounts for 20-30%. We need to pay more attention to the understanding of medical staff and patients, and to R&D personnel. The selection and understanding of government and regulatory regulations.
Professor Yan Zhuangzhi, director of the Shanghai Institute of Biomedical Engineering, has brought in the implementation steps of the new medical reform program and some specific programs. For example, he is stepping up the research on the wireless service platform for long-term non-invasive monitoring of vital signs to cope with the national unified establishment of the new medical reform. The process of resident health records. However, Professor Yan Zhuangzhi also said that it is still a long time since the mature application of the system is being implemented. The implementation of the new medical reform is also facing challenges such as lack of standardization, economic and environmental impact, information security technology, data analysis management and medical manufacturing technology. The road to medical reform still has a long way to go.
Indeed, the implementation of the new medical reform program is inseparable from the leadership of the government and industry organizations, and is inseparable from the strong support of research institutions and production enterprises. At CMET2009, almost all the speakers mentioned how to enhance the interoperability of information between instruments and how to design products that are more in line with market demand for low power consumption, miniaturization, wireless, networking and remoteness. And other issues.
New Technology and New Market Conference Focuses on Five Hotspots in the Industry
Compared with the above-mentioned expert scholar Takagi Kenzo's speech, the information brought by Intel, ADI, XILINX, TI, ACTEL, Wind River System and EVOC Group for CMET2009 participants is more practical. According to them, portable, informative, low Power consumption, low cost, and security are the hottest technology hotspots in the medical electronics industry today.
Portable: Needless to say, the most important theme of this year's China International Medical Electronics Technology Conference is portable, and all the topics on the scene are centered around portable medical electronics. General Manager Li Shuzhen pointed out that after years of continuous innovation and revolution in semiconductor technology, more and more traditional medical measuring instruments are moving toward a portable path, and instruments that have become portable products will be further reduced. The shrinking size of medical electronic products is inseparable from the rapid development of semiconductor technology. ADI, XILINX, TI, ACTEL and other companies have brought products and technology platforms more suitable for portable medical electronics.
Informatization: The trend of informationization is changing the mode of medical treatment, becoming the best way to balance medical equipment and medical resources, ease the difficulty of seeing a doctor, and improve medical safety. Huang Qingchun, Business Development Manager from Intel Digital Healthcare Group, presents mobile clinical assistants based on Intel® Mobile Clinical Assistant (MCA) technology, as well as integrated digital hospitals and rural medical information solutions. ADI, TI, and EVOC Group also showcased products and technologies for medical informationization trends. In the future rural medical information station, the village medical special computer with preset medical auxiliary diagnosis and treatment software will be included, and the new rural cooperative management system can be obtained at any time to obtain the latest medical technology training information, providing rational drug information, chronic disease system diagnosis and treatment technology, and six-in-one. Health management system, patient information collection and management system, and large hospital expert remote diagnosis and consultation system.
Future rural medical information platform
Low power consumption: Portability means that the product will put more demand on standby time. At CMET2009, the MSP430 microcontroller business development experience from TI demonstrated the amazing power consumption characteristics of the MSP430 family: the bio-battery made of only three small apples can drive the MSP430 to work normally for one to two weeks. !
The FPGA vendors Xilinx and Actel, which are mainly pushing low-power features, will certainly not show weakness. Xi Wenxin, the emerging business development manager of Xilinx Asia Pacific, and Dai Menglin, Actel technical support/training manager, respectively, bring ultra-low-power FPGA products and their portable medical devices. In addition to the low power consumption of the FPGA itself, Actel has also introduced comprehensive power analysis and optimization software, including creating power distribution reports, reviewing peak power per cycle and averaging throughout the simulation. Power consumption and other functions.
In addition, Yong Yong also reminds the whole machine manufacturer: It is very simple to reduce a certain index of product power consumption, but the real low-power system design should take into account various states such as standby, power-down protection, and full-speed operation of the machine. In addition, when choosing low-power products, you must keep your eyes open. Many manufacturers like to take power consumption in a specific environment of the product during the promotion. In fact, the power consumption may be multiplied by replacing an environment! 
Low cost: Cost reduction is a must for any market to develop after a certain period of time. The cost of a product can be divided into research and development costs, time cost and manufacturing cost. At this CMET conference, ADI, TI, Xilinx, Actel, Wind River Systems, and EVOC Group all mentioned low cost, and the way companies reduce costs is different. Compared with ADI, TI, Xilinx and Actel, Wind River Systems and EVOC Group offer two other ways to reduce costs by increasing IC integration, reducing peripheral components and reducing overall system cost: EVOC Group Medical Products Line Products Manager Wang Xia said that the use of EVOC's mature system architecture can significantly shorten the time for customers to develop products, especially when the system is upgraded and replaced, it can be replaced by sub-modules, and the lower-generation modules can still be replaced at the low end. The product is used; Fenghe system starts with the operating system and software of the product. Hao Meng, the technical customer manager of Wind River System, introduces the operating system and hardware architecture of the medical electronic product through the unique hypervisor technology of Wind River System. Virtualizes a single hardware system (such as CPU, memory, etc.) into multiple hardware systems and runs multiple software systems in time-sharing mode. By using this method, the functional innovation of the product can be performed without changing the hardware design, which can greatly accelerate the time-to-market of the product. For example, if the new product does not change the hardware architecture, it will no longer need to carry out a variety of demanding testing and certification, so the cost savings will be very large.
Safety: Dr. Wang Xiaoqing pointed out that medical electronics need to pay more attention to providing safety design than ordinary instruments. Medical electronic products must comply with general safety standards such as GB9706 and IEC60601 and special safety standards for specific products, and should also include EU CE certification. And international market access specifications such as the US FDA certification and so on. Actel reminds everyone that in today's IC process is getting smaller and smaller, the number of solid errors caused by charged particles is not serious, especially in the field of medical electronics related to personal safety, how to avoid solid errors It has become a problem that must be considered, and the Flash process FPGA product provided by Actel is one of the best choices for medical electronic design.
Dr. Wang Xiaoqing also said that the design of medical electronics needs to focus on the stability and reliability design of layout and layout in circuit design while applying mature technology, and the components are demanded according to the specific requirements of specific applications. Selection. Therefore, for medical electronic solution providers, understanding and grasping the relevant standards, certifications and specifications of the medical electronics industry and implementing them throughout the design and management of the solution, thereby achieving comprehensive security, is far more than increasing the functionality of the product. More competitive and strategic.
As the only professional forum focusing on medical electronic technology and solutions in China, CMET2009 not only invited international medical electronic core technology and system providers such as TI, ADI, Actel, Xilinx, Intel, Wind River Systems, EVOC Group, etc. Li Shuzhen, general manager of Di Consulting Semiconductor Research Center, Dr. Wang Xiaoqing, member of the Expert Committee of China Medical Device Industry Association, and Professor Yan Zhuangzhi, director of the Institute of Biomedical Engineering of Shanghai University, gave speeches and attracted more than 300 people from Shenzhen Mindray and Jin. Kewei, Guangdong Bao Laite, Libang, Shangrong, Lanyun, Zhuhai and Jia, and other major domestic medical electronic equipment manufacturers, as well as managers and technicians of medical electronic equipment ODM/OEMs such as Flextronics and BYD attended the meeting.
Disposable Medical Surgical Mask Making Machines
Ear Loop Face Mask Making Machine,Medical Face Mask Making Machine,Automatic Medical Face Mask Machine
Shandong Carved Intelligent Technology Group Co.,Ltd , https://www.demetdent.com