Medical Network July 20th In 1986, the world's first murine monoclonal antibody, Muromonab OKT3, was introduced. So far, the monoclonal antibody has developed into the fourth generation of fully humanized monoclonal antibody. The humanized monoclonal antibody can avoid the murine monoclonal antibody response and increase the biological activity of the monoclonal antibody molecule. In view of the most advanced fourth-generation monoclonal antibody, this paper takes stock of domestic and international products, and briefly analyzes the product pipelines of major domestic monoclonal antibody research and development enterprises.
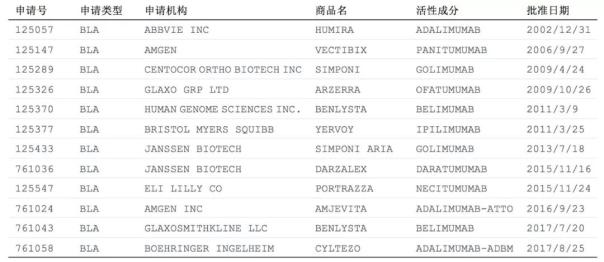
The fourth-generation fully humanized monoclonal antibody US market situation: FDA approved a total of 11 four-generation monoclonal antibody drugs
As of July 2018, 11 humanized monoclonal antibody drugs have been approved for marketing in the US FDA, and 2 of them are biosimilar drugs. The first humanized monoclonal antibody approved in the FDA was the well-known adalimumab (ADALIMUMAB), trade name HUMIRA (Chinese: Xiu Meile), produced by AbbVie, based in Chicago, USA. The approved time is at the end of 2002. HUMIRA is used in the treatment of rheumatoid arthritis. The manufacturer Aberdeen's financial report shows that HUMIRA's global sales in 2017 reached a staggering $18.4 billion, an increase of $2.69 billion from the previous year, a growth rate of 17%, for the sixth consecutive year. Ranked first in the global drug sales list. In 2016, Amgen's biosimilar drug AMJEVITA was approved for marketing at the FDA, which is the first biosimilar drug of adalimumab. In 17 years, Boehringer Ingelheim's CYLTEZO was approved by the FDA to become the second approved adalimumab biosimilar.
The newly approved fourth-generation all-humanized monoclonal antibody product is the biological analogue Cyltezo produced by Boehringer Ingelheim, which was approved on August 25, 2017. Cyltezo is a biosimilar drug for the treatment of autoimmune diseases such as rheumatoid arthritis.
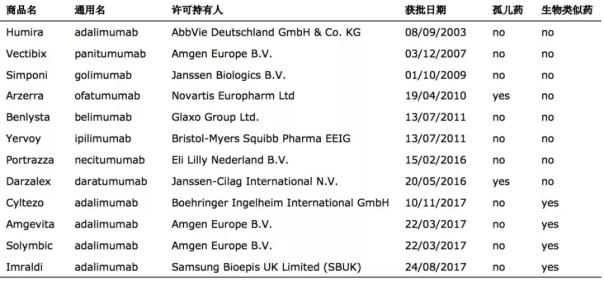
Fourth-generation all-humanized monoclonal antibody EU listing: a total of 12 four-generation monoclonal antibodies listed
As of July 2018, a total of 12 four-generation monoclonal antibodies (excluding two withdrawals) were approved for marketing in the European Union, and four of them were biological analogues. It can be seen that although the number of fourth-generation monoclonal antibodies approved by the EU is higher than that of the United States, the number of newly listed drugs is not as good as that of the United States.
Aberdeen's adalimumab was approved in the European Union in 2002 and was approved in the EU in mid-2003. It is worth noting that among the 12 approved four-generation monoclonal antibodies, there are 2 orphan drugs. Ofatumumab was originally developed by Genmab and was approved for marketing in the US FDA in October 2009. It was approved by the European Union in 2010 and obtained orphan drug identification. It is a drug for the treatment of chronic lymphocytic leukemia.
   Table: EU-approved fourth-generation monoclonal antibody
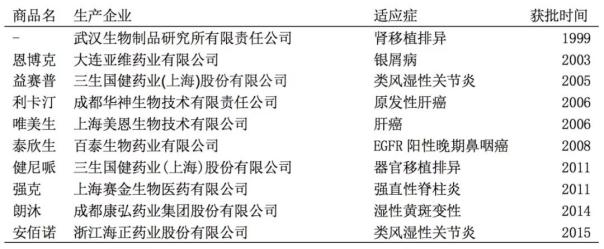
Domestic monoclonal antibody market situation: a total of 28 monoclonal antibody drugs were approved, 10 domestically produced drugs
According to the results of the CFDA inquiry, as of July 2018, 28 domestic monoclonal drugs (including fusion proteins) were approved, including 10 domestic monoclonal antibody drugs and 18 imported monoclonal antibody drugs.
On June 15, 2018, Navulubib injection (English name: Nivolumab Injection) was officially approved by the CFDA. This is the first PD-1 monoclonal antibody marketed in China.

Domestic enterprise monoclonal antibody drug layout
Excluding fusion protein, only 6 domestic monoclonal antibody varieties are currently listed in China. In the United States, only the fourth generation of monoclonal antibodies has 11 products listed, and the EU has approved 4 fourth-generation monoclonal antibodies, the gap is obvious. Among the listed companies, Sansheng Pharmaceutical (01530.hk), Taihe Health (000790.sz), Kanghong Pharmaceutical (002773.sz), and Hisun Pharmaceutical (600267.sh) have listed monoclonal antibodies; we select domestic Some companies with strong R&D strength in the field of monoclonal antibodies do a brief inventory of their main research varieties.
Fu Hong Hanlin
Shanghai Fuhong Hanlin Biotechnology Co., Ltd., a subsidiary of Fosun Group, was established in December 2009 by Shanghai Fosun Pharmaceutical (Group) and Hanlin Biopharmaceutical Company. Fuhong Hanlin is a top-ranking company in China's monoclonal antibody research and development. It is rich in research pipelines, including bio-analogs, modified monoclonal antibodies, innovative monoclonal antibodies, bispecific monoclonal antibodies and antibody-conjugated drugs.
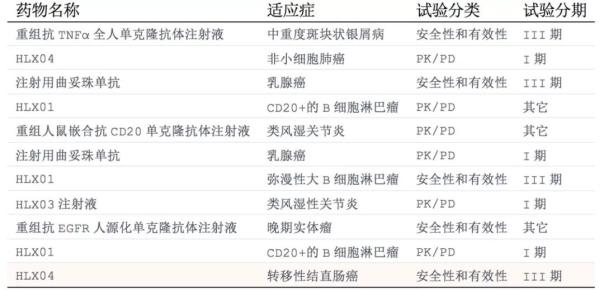
The fastest progress of the company's variety is rituximab, which was submitted for listing at the end of 2017. Other varieties such as trastuzumab-like drugs, research and development of adalimumab-like drugs are also advancing. CDE data shows that there are currently 11 clinical trials in the company, of which 4 trials have entered clinical phase III.
Table: Fuhong Hanlin's clinical trial report on drugs
Sansheng Guojian (a wholly-owned subsidiary of Hong Kong stock Sansheng Pharmaceutical)
Sansheng Guojian was established in 2002 and currently has two benefits: Saip (recombinant human type II tumor necrosis factor receptor antibody fusion protein for injection) and Jiannipei (recombinant anti-CD25 humanized monoclonal antibody injection). Anti-drugs are listed. Yisaipu is the first fusion protein drug listed in China, with a market share of over 90%.
Jiahe Bio
Founded in 2007, Jiahe Bio is an innovative and driven international biopharmaceutical company. It was originally a subsidiary of the listed company Watson Bio (300142). After the equity transfer, Watson's shareholding ratio fell to 13.59%. The company is mainly engaged in the research and development and transformation of monoclonal antibody, Fc-fusion protein drugs and other monoclonal antibodies, and currently has more than 10 varieties. At present, the fastest progress of GB222 in the company's research varieties is a recombinant anti-vascular endothelial growth factor humanized monoclonal antibody injection for non-small cell lung cancer treatment, which is already in clinical phase III.
Kang Hong Pharmaceutical
Compaqip Eye Injection (commercial name Langmu) is a well-known variety of Kanghong Pharmaceutical. It was launched in 2013, with sales of 640 million in 2017, a year-on-year growth rate of 35%.
Compaqip was included in the WHO generic drug list in 2012 and became the first class 1 biologics in China to enter the WHO INN List. At present, Compaqip has two indications approved: wet age-related fundus macular degeneration (wAMD) and choroidal neovascularization (pmCNV) secondary to pathological myopia.
At present, Kanghong Pharmaceutical's research pipeline mainly includes three products, KH902, KH903 and KH906. Its main components are Compaqip. KH902 is the Compaqip Eye Injectable Solution, which is on the market and is currently undergoing clinical research on other indications. KH903 injection is used to treat rectal cancer, and KH906 eye drops are used to treat corneal neovascularization.
Cinda Creature
Founded in 2011, Cinda Bio is a new monoclonal antibody company that develops, manufactures and markets monoclonal antibodies for the treatment of major diseases such as cancer. Cinda Biotech submitted a Hong Kong stock prospectus on June 28, 2018, becoming the third biotech company to apply for listing without a revenue.
At present, the company has 4 products entering the third phase of clinical, and 2 selected into the national “major new drug creation†technology major project. The anti-CD20 monoclonal antibody injection developed by the company is a biosimilar drug of rituximab, the world's best-selling monoclonal antibody, for the treatment of non-Hodgkin's lymphoma. It is currently in Phase III clinical stage. The anti-TNF-α monoclonal antibody injection is a biosimilar drug of adalim, and has now entered the phase III clinical research stage. The other two biosimilars that entered the phase III clinical trial included anti-VEGF monoclonal antibody injections and anti-PD-1 monoclonal antibody injections.
Veterinary Drugs: refers to substances (including medicated feed additives) used to prevent, treat, diagnose animal diseases or purposefully regulate animal physiological functions.
Veterinary drugs mainly include: serum products, microecological products, Chinese herbal medicines, proprietary Chinese medicines, chemicals, antibiotics, and topical pesticides, disinfectants, etc.

Veterinary Drugs,Veterinary Medicine,Veterinary Injectable Drugs,Veterinary Pharmaceutical Drugs
XI AN RHINE BIOLOGICAL TECHNOLOGY CO.,LTD , https://www.rhinebiotech.com