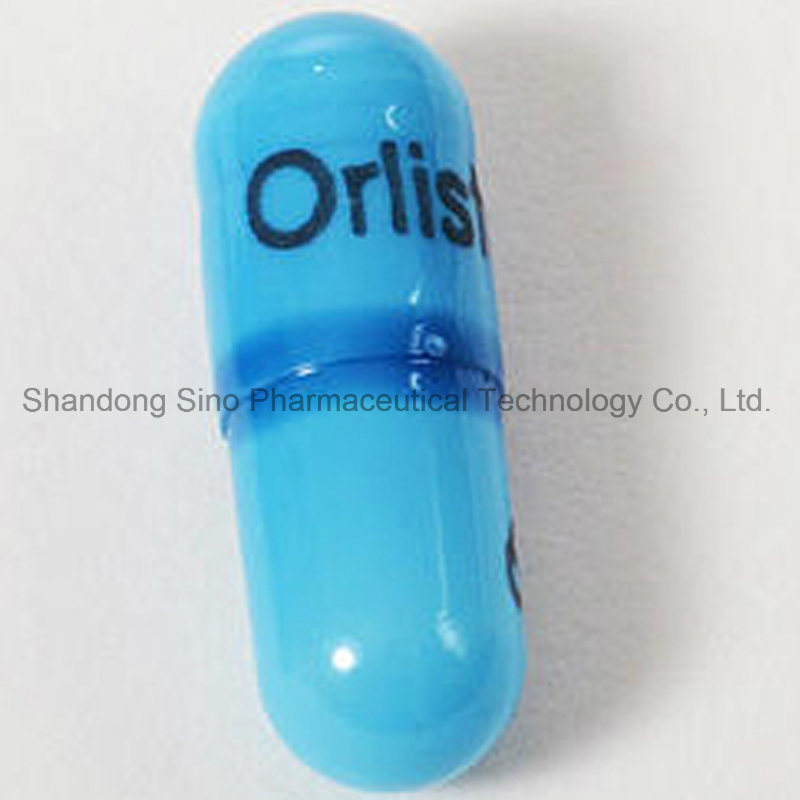
| PRODUCT NAME: | Venical® (Orlistat Capsules ) |
| STRENGTH: | 60mg, 120mg |
| PACKING DETAILS: | 42 capsules/box, 84 capsules/box |
| STORAGE: | Store in a cool and dry place below 25ºC, protected from light. |
| SHELF LIFE: | 36 months |
| REGISTRATION DOSSIERS ARE AVAILABLE. | |
| CONSIGNMENT MANUFACTURING, BRAND OEM/ODM SERVICE IS AVAILABLE. | |


1. Indication
Venical is indicated in conjunction with a mildly hypocaloric diet for the treatment of obese patients with a body mass index (BMI) greater or equal to 30 kg/m², or overweight patients (BMI > 28 kg/m²) with associated risk factors.
Treatment with orlistat should be discontinued after 12 weeks if patients have been unable to lose at least 5 % of the body weight as measured at the start of therapy.
Â
Adults
The recommended dose of orlistat is one 120 mg capsule taken with water immediately before, during or up to one hour after each main meal. If a meal is missed or contains no fat, the dose of orlistat should be omitted.
The patient should be on a nutritionally balanced, mildly hypocaloric diet that contains approximately 30 % of calories from fat. It is recommended that the diet should be rich in fruit and vegetables. The daily intake of fat, carbohydrate and protein should be distributed over three main meals.
Doses of orlistat above 120 mg three times daily have not been shown to provide additional benefit.
The effect of orlistat results in an increase in faecal fat as early as 24 to 48 hours after dosing. Upon discontinuation of therapy, faecal fat content usually returns to pre-treatment levels, within 48 to 72 hours.
Â
Special populations
The effect of orlistat in patients with hepatic and/or renal impairment, children and elderly patients has not been studied.
There is no relevant indication for use of Venical in children.
Â
- Hypersensitivity to the active substance or to any of the excipients.
- Chronic malabsorption syndrome.
- Cholestasis.
- Breast-feeding.
4. Undesirable Effects:
Adverse reactions to orlistat are largely gastrointestinal in nature. The incidence of adverse events decreased with prolonged use of orlistat.
Adverse events are listed below by system organ class and frequency. Frequencies are defined as: very common (≥1/10), common (≥1/100 to <1/10), uncommon (≥1/1,000 to <1/100), rare (≥1/10,000 to <1/1,000) and very rare (<1/10,000) including isolated reports.
5. Overdosage:
Single doses of 800 mg orlistat and multiple doses of up to 400 mg three times daily for 15 days have been studied in normal weight and obese subjects without significant adverse findings. In addition, doses of 240 mg tid have been administered to obese patients for 6 months. The majority of orlistat overdose cases received during post-marketing reported either no adverse events or adverse events that are similar to those reported with recommended dose.
Should a significant overdose of orlistat occur, it is recommended that the patient be observed for 24 hours. Based on human and animal studies, any systemic effects attributable to the lipase-inhibiting properties of orlistat should be rapidly reversible.
6. Presentation
-42 capsules/box or 84 capsules/box
Marketing Authorisation Holder:
Laboratories Viroche S.A.Â
All Designed and formulated from France
Global Supplier and Exporter:
Shandong Sino Pharmaceutical Technology Co.,Ltd.
North of National Road 327, Liuhang Town, High-tech Zone, Jining, China.
Payment: Â T/T or west union
Delivery time: within 2 working days for ready stocks, 45 working days for batch order.
Shipment: HKEMS,TNT,UPS,door to door service for some countries.
We are looking for the VENICAL® agents all around the world.
Welcome to join us!
Moreover, kindly note that we are always providing our customers with Consignment Manufacturing Serivce, OEM/ODM.
Brief Introduction to Our Company:
Shandong Sino Pharmaceutical Technology Co., Ltd. (S.S.P.T.)  is a different kind of pharmaceuticals (both finished medicine products and raw materials), health products, cosmetics and medical supplies company for human needs, focusing on global markets, especially the developing world. Working with the highest levels of government and local distributors alike, we aim to not only expand our business scale and scope but also have a special interest in serving the needs of the end consumer that requires our products the most. To this end, we have a presence in 5 continents with an expanding product range to meet the demand of our main markets.
Â
Moreover, our manufacturing facilities follow strict GMP rules and regulations. We have intergraded all GMP guidelines into internal procedures improving the degree to which results are consistently exceeding expectations. As one of the best pharmaceutical companies in China, we take our ethical obligation to improving access to high quality and affordable WHO essential drugs seriously. This is the ethical basis our company was founded on.
Consignment Manufacturing, Brand OEM/ODM Service are available.
Â
| PRODUCT NAME: | Venical® (Orlistat Capsules ) |
| STRENGTH: | 60mg, 120mg |
| PACKING DETAILS: | 42 capsules/box, 84 capsules/box |
| STORAGE: | Store in a cool and dry place below 25ºC, protected from light. |
| SHELF LIFE: | 36 months |
| REGISTRATION DOSSIERS ARE AVAILABLE. | |
| CONSIGNMENT MANUFACTURING, BRAND OEM/ODM SERVICE IS AVAILABLE. | |





1. Indication
Venical is indicated in conjunction with a mildly hypocaloric diet for the treatment of obese patients with a body mass index (BMI) greater or equal to 30 kg/m², or overweight patients (BMI > 28 kg/m²) with associated risk factors.
Treatment with orlistat should be discontinued after 12 weeks if patients have been unable to lose at least 5 % of the body weight as measured at the start of therapy.
Â
Adults
The recommended dose of orlistat is one 120 mg capsule taken with water immediately before, during or up to one hour after each main meal. If a meal is missed or contains no fat, the dose of orlistat should be omitted.
The patient should be on a nutritionally balanced, mildly hypocaloric diet that contains approximately 30 % of calories from fat. It is recommended that the diet should be rich in fruit and vegetables. The daily intake of fat, carbohydrate and protein should be distributed over three main meals.
Doses of orlistat above 120 mg three times daily have not been shown to provide additional benefit.
The effect of orlistat results in an increase in faecal fat as early as 24 to 48 hours after dosing. Upon discontinuation of therapy, faecal fat content usually returns to pre-treatment levels, within 48 to 72 hours.
Â
Special populations
The effect of orlistat in patients with hepatic and/or renal impairment, children and elderly patients has not been studied.
There is no relevant indication for use of Venical in children.
Â
- Hypersensitivity to the active substance or to any of the excipients.
- Chronic malabsorption syndrome.
- Cholestasis.
- Breast-feeding.
4. Undesirable Effects:
Adverse reactions to orlistat are largely gastrointestinal in nature. The incidence of adverse events decreased with prolonged use of orlistat.
Adverse events are listed below by system organ class and frequency. Frequencies are defined as: very common (≥1/10), common (≥1/100 to <1/10), uncommon (≥1/1,000 to <1/100), rare (≥1/10,000 to <1/1,000) and very rare (<1/10,000) including isolated reports.
5. Overdosage:
Single doses of 800 mg orlistat and multiple doses of up to 400 mg three times daily for 15 days have been studied in normal weight and obese subjects without significant adverse findings. In addition, doses of 240 mg tid have been administered to obese patients for 6 months. The majority of orlistat overdose cases received during post-marketing reported either no adverse events or adverse events that are similar to those reported with recommended dose.
Should a significant overdose of orlistat occur, it is recommended that the patient be observed for 24 hours. Based on human and animal studies, any systemic effects attributable to the lipase-inhibiting properties of orlistat should be rapidly reversible.
6. Presentation
-42 capsules/box or 84 capsules/box
Marketing Authorisation Holder:
Laboratories Viroche S.A.Â
All Designed and formulated from France
Global Supplier and Exporter:
Shandong Sino Pharmaceutical Technology Co.,Ltd.
North of National Road 327, Liuhang Town, High-tech Zone, Jining, China.
Payment: Â T/T or west union
Delivery time: within 2 working days for ready stocks, 45 working days for batch order.
Shipment: HKEMS,TNT,UPS,door to door service for some countries.
We are looking for the VENICAL® agents all around the world.
Welcome to join us!
Moreover, kindly note that we are always providing our customers with Consignment Manufacturing Serivce, OEM/ODM.
Brief Introduction to Our Company:
Shandong Sino Pharmaceutical Technology Co., Ltd. (S.S.P.T.)  is a different kind of pharmaceuticals (both finished medicine products and raw materials), health products, cosmetics and medical supplies company for human needs, focusing on global markets, especially the developing world. Working with the highest levels of government and local distributors alike, we aim to not only expand our business scale and scope but also have a special interest in serving the needs of the end consumer that requires our products the most. To this end, we have a presence in 5 continents with an expanding product range to meet the demand of our main markets.
Â
Moreover, our manufacturing facilities follow strict GMP rules and regulations. We have intergraded all GMP guidelines into internal procedures improving the degree to which results are consistently exceeding expectations. As one of the best pharmaceutical companies in China, we take our ethical obligation to improving access to high quality and affordable WHO essential drugs seriously. This is the ethical basis our company was founded on.
Consignment Manufacturing, Brand OEM/ODM Service are available.
Â
Small Air Hose Reel,Portable Air Hose Reel,Wall Mount Air Hose Reel,Commercial Air Hose Reel
NINGBO QIKAI ENVIRONMENTAL TECHNOLOGY CO.,LTD , https://www.water-hose-reel.com