Release date: 2016-10-17
Since the final version of the FDA's Mobile Medical Application Final Guidance in September 2013, it has been monitoring digital health products like regulatory medical devices. Although entrepreneurship in the digital health field has been raging, how many of them can pass the FDA exam? The FDA's management of medical devices is performed through the Center for Devices and Radiological Health (CDRH). CDRH is a division of the FDA that specializes in the approval of medical devices, including the digital health products of the APP.
Looking at the digital health products approved by the center in the third quarter of 2016, we will see what the FDA's eye-qualified digital health products look like. Most of the equipment or apps approved by the FDA in the third quarter were for diabetes management. Of the seven license management, only two were for other health issues. Among the seven digital health products, there is only one as a stand-alone app.
Medallion
Medical device giant Medtronic has two seats. The two devices approved by Medtronic are in the field of diabetes. The first is an FDA-approved hybrid closed-loop insulin delivery system, the MiniMed 670G and the Sugar.IQ app (the latter was developed in conjunction with IBM Watson). It is worth mentioning that Medtronic's MiniMed 670G hybrid closed-loop system is the world's first device to help patients continuously monitor blood sugar, and automatically introduce insulin as needed, known as "artificial pancreas."
The entire equipment system consists of a drug pump of the size of a deck of cards, a sensor for measuring blood sugar, and a tube that supplies insulin. After being “bound†to the patient, the MiniMed 670G monitors the sugar glucose every 5 minutes. If an abnormality occurs, the system will automatically adjust and inject a dose of insulin to ensure that the user's blood sugar is in a healthy range.
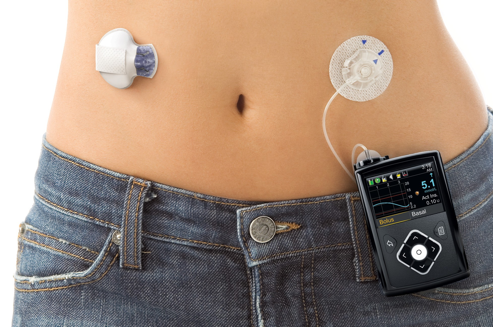 ↑MiniMed 670G
↑MiniMed 670G
At the same time, Medtronic's Guardian Connected Mobile Continuous Blood Glucose Monitoring (CGM) and app also received the EU CE mark, which is expected to be available in selected countries in Europe, Asia and Latin America in the second quarter of 2017. The system, and they also hope to get FDA approval in the US to start the system, Medtronic now has an FDA-approved and CE-markable connectable CGM system (named Minimed Connect), but the system is suitable for Medtronic CGM and insulin. The user of the pump.
Ascensia that allows Chinese companies to sprinkle salt
Remember the news that the Chinese company Sannuo Bio was bidding for Bayer's blood glucose meter business last year? This product is the news of salting Sannuo. Ascensia Diabetes Care (Ascensia Diabetes Care) is a new business unit that defeated Sanno Biotech's acquisition of Bayer's Diabetes Care Group's Panasonic Medical Group. Today, its new generation of blood glucose monitoring system (Contour Next Link blood glucose monitor) has announced the launch of the FDA. 510(k) applied. Contour is a blood glucose monitor with fingertips, and Contour Next Link is a new version that transmits data wirelessly to Medtronic's MiniMed 630G insulin pump. According to Ascensia, Contour Next Link is currently The only blood glucose meter that has been approved by the FDA to be connected to an insulin pump.
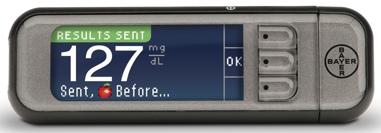

Lilly and son
Innovations in the delivery of diabetes drugs have never stopped. Insulin pens and related apps developed by Companion Medical of San Diego, Calif., have just passed FDA approval in July. The first FDA-approved smart insulin pen can be used in Eli Lilly's best-selling drug, Humalog. And Novo's best-selling insulin drug, NovoLog, allows patients to track and calculate insulin measurements while sending information to health care workers.

It is worth mentioning that Companion Medical took a round of Lilly's investment last year.
We finally use the equipment first than the Americans.
Why do you always use foreign things first? Don't be discouraged, this device is better than the Americans.
On September 29th, Abbott Instant Dynamic Glucose Doctor Professional Edition (FreeStyle Libre Pro) received US FDA certification. The transient was approved by the CFDA on August 29, just one month before the FDA. In fact, in Europe, the Abbott Instant Blood Glucose Meter has been recognized by the CE mark in September 2014, and it currently has both prescription and over-the-counter versions, which are also compatible with mobile phones; the effect of the FreeStyle Libre Pro system is very significant because It does not require a hand-pear to calibrate, and it is less expensive than other CGMs on the market, and users only need to carry a small pluggable sensor and patch on their arms for 14 days. The patch records the blood glucose data of the patient's body every 15 minutes and uses NFC technology (near-field communication technology) to transmit data.
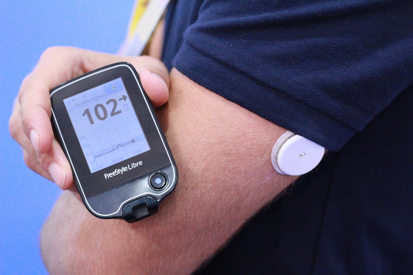
↑FreeStyle Libre Pro
It should be noted that the FDA license is the doctor's professional version of the Instant Dynamic Blood Glucometer FreeStyle Libre Pro, and the patient version of the Instantaneous FreeStyle Libre? Sorry, the FDA is still evaluating it.
G2 of "Second Palace"
On August 25th, the FDA passed the first all-weather portable blood glucose monitor, Dexcom's G5 Mobile CGM System. An advisory panel from the FDA has now voted in favor of changing the intended use of the G5 continuous diabetes monitoring system. What does it mean? In fact, G5 has been approved by the FDA before, but from a detail we can still see the rigor and rigor of FDA certification: At the time, the approval of G5 was based on a series of restrictions as an auxiliary device. This means that it can only be used as a system for tracking and turning to the glucose concentration in the interstitial fluid, rather than as a fast blood glucose meter (BGM) that completely replaces the traditional finger thorn.

This time the FDA follows the advice of the advisory panel, then Dekang Medical will bring the G5 system to market as a basic therapy decision for patient treatment, not just BGM supplementation, in order to support the potential of the G5 system. Uses, prior to the FDA meeting, Dekang Medical provided data on the G4 CGM clinical trial.
By embedding wireless Bluetooth technology on the sensor, the G5 mobile blood glucose monitoring system can send the test data directly to the app on the iOS system phone. The Android app is expected to be available early next year. Dexcom says the device is not only suitable for adults, it is said to be available even for children as young as 2 years old.
Shameful APP: PeriCoach
Having said so many devices for diabetes, we have been "greasy" to no. Fortunately, the FDA is not only sweet.
PeriCoach, developed by Australian company Analytica, is a mobile app for Kegel training. The so-called "Kegel Training Method" was developed in 1948 by Dr. Arnold Kegel for the treatment of urinary incontinence - by exercising the shame-tailbone muscle group for the purpose of increasing urethral resistance; he later discovered that this method Not only can the tension of the pelvic muscles be restored, but also the genital area can be stimulated to increase blood flow, thereby improving sexual function.

PeriCoach is such an app that helps girls do shame and exercise. In July this year it received a second FDA 510(k) approval to make it available as an over-the-counter device. The FDA approved it as a prescription device for sale in March last year. PeriCoach's pricing is not cheap, and the subscription fee for a year is $299.
苹果上Apple's AirStrip
AirStrip's TeleStrip Remote Patient Monitoring solution (RPM) was approved by the FDA at the end of July this year.
AirStrip is well known to everyone thanks to Apple. In 2015, AirStrip appeared at Apple's new product launch. The AirStrip app allows doctors to remotely view patient heart rate data via iPad or iPhone, which is now available for Apple Watch. The company has also developed a system that can help doctors monitor the fetal heart of high-risk pregnant women.

The AirStripOB developed by the company is also one of the first medical applications in the FDA-approved Apple App Store. It is said that AirStrip is already preparing for an IPO.
Source: Health Point
Organic grape seed extract is, as the name implies, extracted from the high-quality organic grape seed. We use an organic extraction solvent, which is extracted in strict accordance with the EU and USDA organic processing specifications. High content OPC extract is not added with any carrier, such as OPC 95% and OPC 98%. The Grape Seed Extract powder is rich in Oligomers Procyanodolic Complexes (OPC), which is a powerful antioxidant. In addition to the ultra-rich potency of over 20 times higher than Vitamin C, it is also 50 times better than Vitamin E. It helps to strengthen the immune system, and also slow down the aging process, which is of very high market value.
Grapeseed Extract,Grapeseed Extract Organic,Organic Grape Seed Extract,Grape Seed Extract Powder
Organicway (xi'an) Food Ingredients Inc. , https://www.organicwayince.com